Email: cspc@cspc.cn
Email: cspc@cspc.cn
Clinical Trials Transparency
September. 21, 2022
The annual meeting of the European Society for Medical Oncology (ESMO) was held in Paris, France. As the most prestigious and influential oncology conference in Europe, the ESMO Congress is a platform for the publication of the latest clinical research findings in the field of oncology.
The findings of a dose-escalation and dose-expansion study of mitoxantrone hydrochloride liposome in combination with pegaspargase for treating ENKTCL (extranodal NK/T-cell lymphoma), led by Professor Huang Yunhong from the Affiliated Hospital of Guizhou Medical University, were presented at the Mini oral session of 2022 ESMO Congress.
Background
Extranodal NK/T-cell lymphoma (ENKTCL) is a subtype of non-Hodgkin's lymphoma with a poor prognosis and a higher incidence in Asian countries than in Europe and the United States. There is still a lack of efficient clinical treatment and an urgent need to be met. Mitoxantrone hydrochloride liposome (PLM60) is the world's first mitoxantrone nanomedicine independently developed by CSPC, which has good anti-tumor activity. This study aimed to evaluate the safety and tolerance, pharmacokinetics (PK), and preliminary efficacy of PLM60 in combination with pegaspargase in treating patients with ENKTCL.
Methodology
Adult patients with histologically confirmed primary or relapsed/refractory ENKTCL were included in this study. A "3+3" dose escalation design was used in Phase I. Four dose levels of PLM60 (12, 16, 20, and 24 mg/m2) combined with pegaspargase 2000 IU/m2 were administered on Day 1 of each cycle (21-day cycle) for a total of 4-6 cycles. Phase II dose expansion was made at the recommended Phase II dose (RP2D) in patients with primary ENKTCL. The primary endpoint was safety, and the secondary endpoints were efficacy based on the Lugano 2014 assessment, including complete response (CR) rate, objective response rate (ORR), and PK.
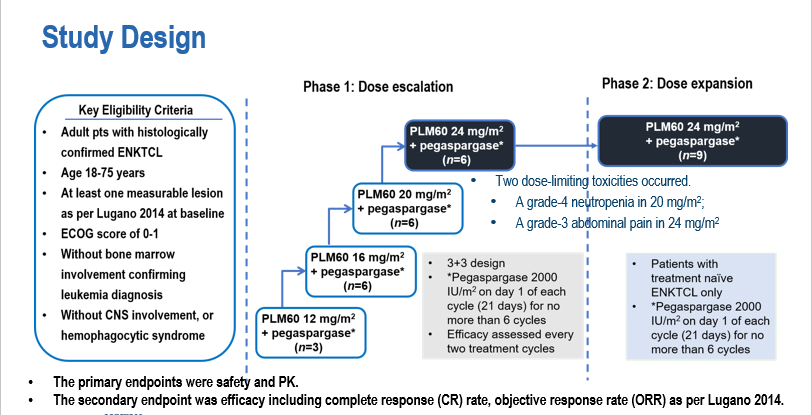
Table 1: Baseline Characteristics of Patients
Findings
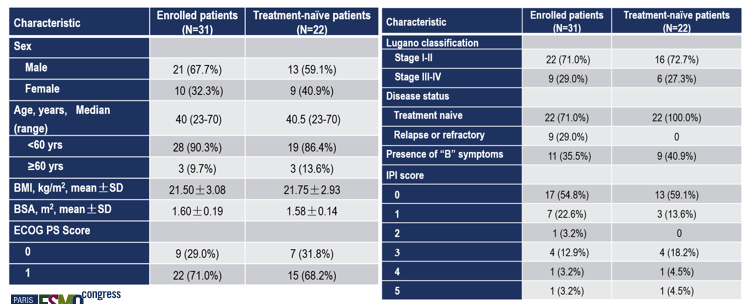
As of February 15, 2022, 31 patients were enrolled (Phase I: 21, Phase II: 10), 9 with relapsed/refractory ENKTCL and 12 with primary ENKTCL in Phase I.
Safety
A total of 2 dose-limiting toxicity events occurred during the dose-escalation phase (1 Grade 4 neutropenia in the 20 mg/m2 group; 1 Grade 3 abdominal pain in the 24 mg/m2 group). RP2D was PLM60 24 mg/m2 + pegaspargase 2000 IU/ m2.
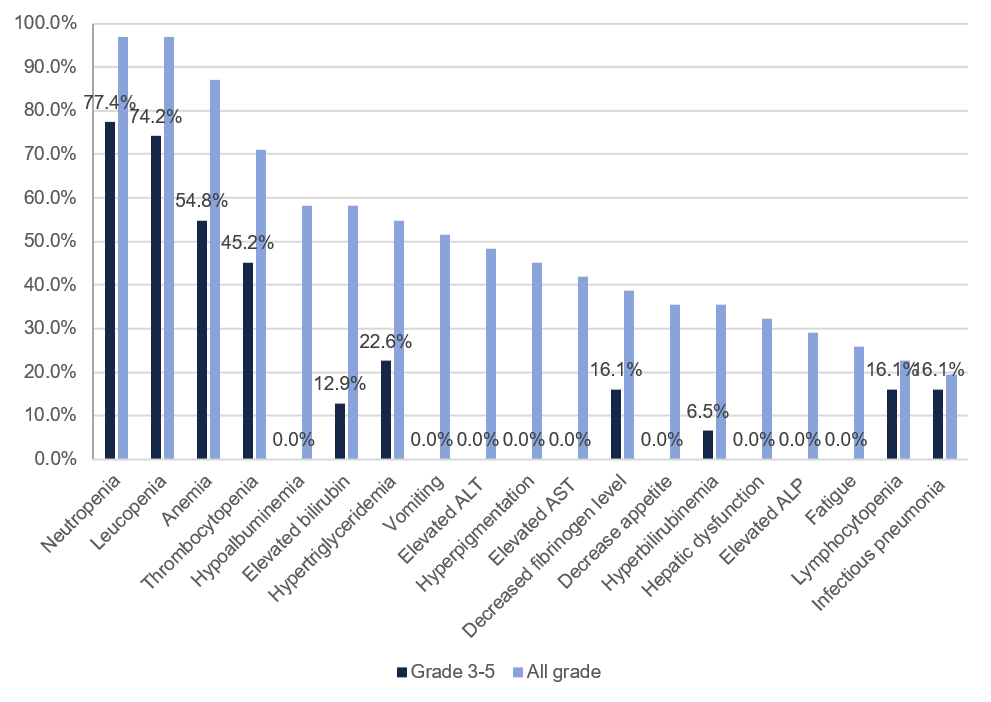
All 31 patients had treatment-related adverse events (TRAEs), of which 27 had Grade 3 or higher TRAEs. The most common Grade 3 and higher TRAEs were neutropenia (77.4%), leukopenia (74.2%), anemia (54.8%), thrombocytopenia (45.2%), hypertriglyceridemia (22.6%), infectious pneumonia (16.1%), lymphopenia (16.1%), decreased fibrinogen (16.1%), hypoglycemia (12.9%) and increased bilirubin (12.9%).
Efficacy
Efficacy was evaluated in all 31 patients, with CR and ORR of 61.3% (19/31, 95% CI 42.2%-78.2%) and 87.1% (27/31, 95% CI 70.2%-96.4%), respectively. Median PFS was not reached.The median age of the 22 patients with primary ENKTCL was 40.5 years (range: 23-70 years); 13 were male, 9 had B symptoms, and 6 had Stage III or IV condition (Lugano staging). The CR rate and ORR for this cohort were 68.2% (15/22, 95% CI 45.1%-86.1%), and 90.9% (20/22, 95% CI 70.8%-98.9%), respectively.
Conclusions
Preliminary results show that PLM60, in combination with pegaspargase, is efficacious, safe, and controllable, especially for patients with primary ENKTCL.