Email: cspc@cspc.cn
Email: cspc@cspc.cn
Clinical Trials Transparency
September. 01, 2022
Recently, a study, conducted by CSPC and the team of Professor Yuan Xianglin from Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, was published in the Therapeutic Advances in Medical Oncology journal (IF: 5.485, JCR Division: Oncology Division 2). It is a multicenter, open, randomized, and controlled clinical study of paclitaxel for injection (albumin-bound) combined with GIO versus SOX regimen for the first-line treatment of inoperable locally advanced, relapsed, or metastatic gastric adenocarcinoma.
Background
Studies have shown the synergistic anti-tumor effects of paclitaxel for injection (albumin-bound) in combination with GIO (AS). In 2016, a multicenter, open-label, dose-escalating study demonstrated that the AS regimen had been well-tolerated by patients with inoperable or relapsed gastric cancer and shown anti-tumor efficacy. This study aimed to compare the efficacy and safety of the AS regimen to standard chemotherapy regimens (oxaliplatin combined with GIO, SOX) in gastric cancer.
Methodology
This randomized, controlled, multicenter clinical study enrolled patients with advanced gastric cancer who had not received prior treatment. The patients were randomized to the AS group [paclitaxel for injection (albumin-bound): 260 mg/m², d1 or 130 mg/m², d1, 8; GIO: 80-120 mg/d, d1~14] or the SOX group (oxaliplatin 130 mg/m2, d1. GIO: 80-120mg/d, d1~14), receiving corresponding treatments every 3 weeks for 6 cycles or until disease progression or intolerable toxicity occurs. The primary endpoint of the study was progression-free survival (PFS), with secondary endpoints including overall survival (OS), objective response rate (ORR), and safety.
Results
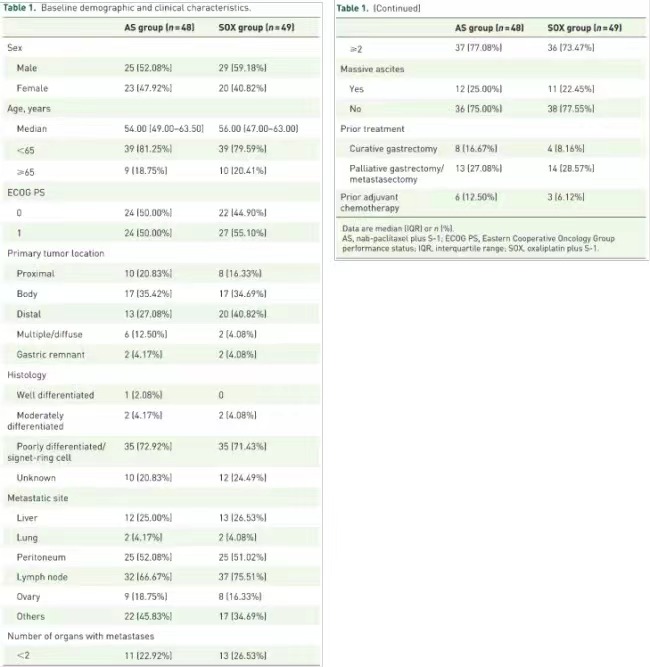
Figure 1 Baseline characteristics of patients
By the time of data cutoff, the median follow-up time for all patients was 23.13 months (95% CI, 13.39-32.87). See Table 1 for the efficacy results.
Endpoint | AS group (n=48) | SOX group (n=49) | P value |
mPFS | 9.03 months (95% CI: 6.05-11.56) | 5.07 months (95% CI: 4.33-5.81) | 0.025 |
mOS | 14.33 months (95% CI: 5.99-22.67) | 15.33 months (95% CI: 6.02-24.64) | 0.604 |
ORR | 39.58% (95% CI: 25.77-54.73) | 32.65% (95% CI: 19.95-47.54) | 0.53 |
DCR | 81.25% (95% CI: 67.37-91.05) | 69.39% (95% CI: 0.240 54.58 81.75) | 0.24 |
Table 1 Efficacy endpoints of the study
The median PFS of the AS group showed a tendency to outperform the SOX group (9.03 months VS. 5.07 months, P=0.025) (Figure 2a). There were no statistical differences in the median OSs (AS: 14.33 months VS. SOX: 15.33 months, P=0.604) (Figure 2b) and ORRs (39.58% VS. 32.65%, P=0.530) between the two groups. Since most patients received subsequent anti-tumor therapy [immune checkpoint inhibitors, paclitaxel for injection (albumin-bound), anti-angiogenic drugs, etc.] after the progression of first-line treatment, it cannot be ruled out that the effect of subsequent therapy on OS resulted in no statistical OS differences between the two groups.
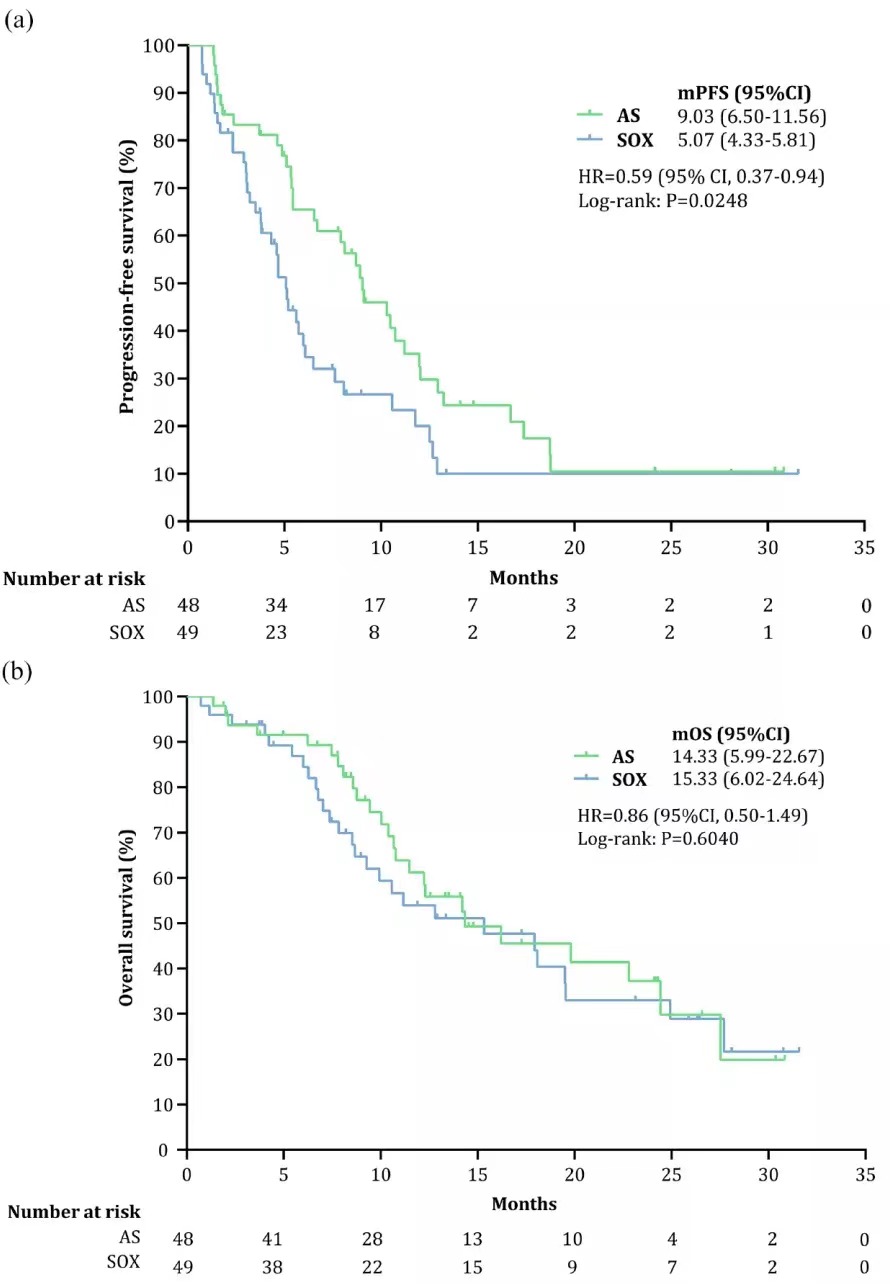
Figure 2 The median PFS and median OS of the AS and SOX groups
Exploratory analysis of PFS showed that most subgroups were more likely to benefit from the AS regimen, especially for patients with poor prognoses, such as scoring 1 in ECOG, poor differentiation, and positive lymph node metastasis (Figure 3). In the multivariate analysis of PFS, chemotherapy regimen, ECOG, and liver metastasis were independent influencing factors, with patients on the AS regimen having a 1.72 times lower risk of disease progression compared to patients on the SOX regimen.
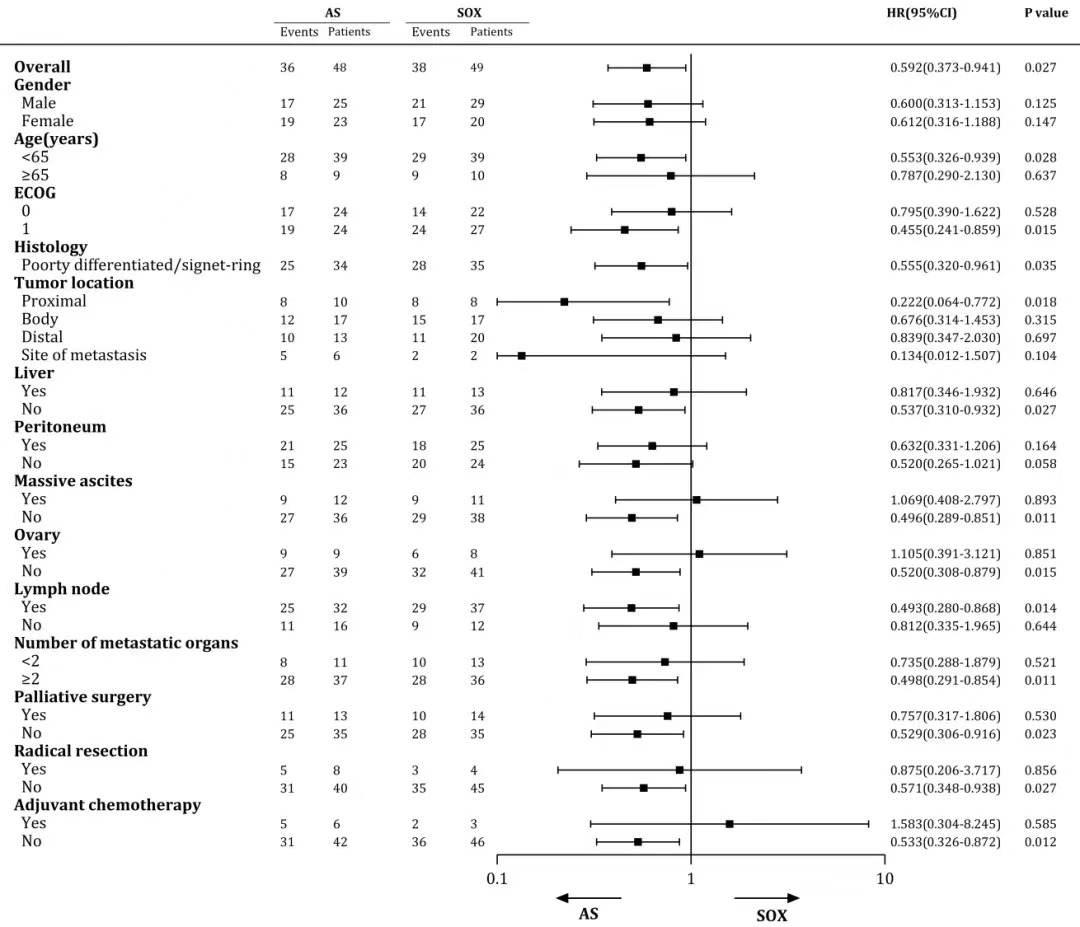
Figure 3 PFS subgroup analysis
Treatment-related adverse events (TRAE) mainly were Grade 1-2 and manageable in both groups. The most common Grade 3~ 4 TRAEs included decreased neutrophil count (AS group VS. SOX group: 20.83% VS. 24.49%), anemia (14.58% VS. 12.24%), decreased red blood cell count (12.50% VS. 12.24%), and decreased white blood cell count (8.33% VS. 16.33%).
Conclusions
This study showed that in the first-line treatment of inoperable locally advanced, relapsed, or metastatic gastric adenocarcinoma, a tendency of benefit was observed in the AS group compared to the SOX group in terms of PFS with manageable toxicity.
References
1.Suenaga M, Yamada S, Fujii T, et al. S-1 plus nab-paclitaxel is apromising regimen for pancreatic cancer in a preclinical model. JSurg Oncol. 2016 Mar;113(4):413-9.
2.Nakayama N, Ishido K, Chin K, et al. A phase I study of S-1 incombination with nab-paclitaxel in patients with unresectable orrecurrent gastric cancer. Gastric Cancer. 2017 Mar;20(2):350-357.